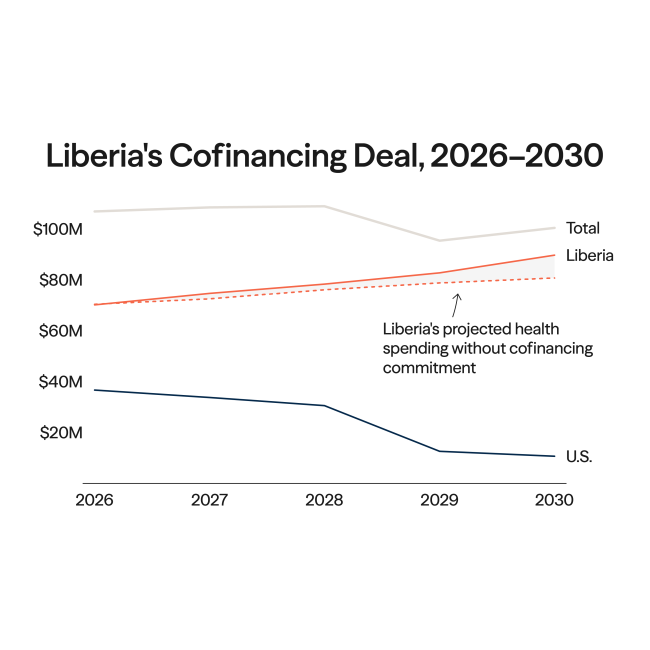
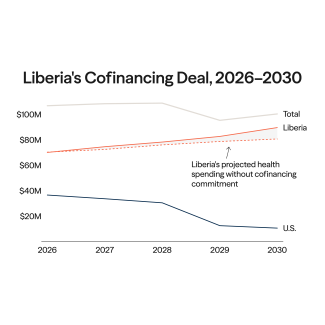
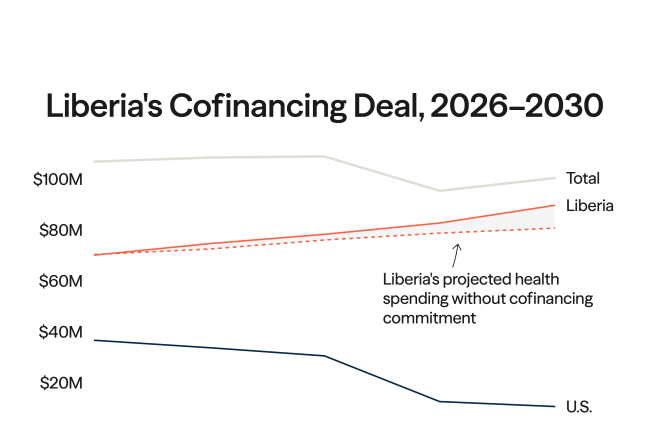
On July 16 and 17, member states of the World Health Organization (WHO) gathered for the tenth round of negotiations on a pandemic agreement. Governments missed their self-imposed deadline to conclude the agreement before the World Health Assembly meeting in May. The Intergovernmental Negotiating Body (INB) resumed the talks as wars, elections, inflation, climate crises, and other issues push pandemics down the global political agenda.
That difficult political context should not, however, obscure the fact that WHO member states made substantial progress toward a pandemic agreement in prior negotiating rounds. The spread of H5N1 avian influenza in dairy cattle in the United States is another reminder that countries urgently need better international rules to mitigate the threat of pandemics. A meaningful pandemic agreement is within reach, but the INB needs to resolve complex and controversial issues, especially the establishment of a pandemic pathogen access and benefit-sharing system.
Has Momentum for a Pandemic Agreement Been Lost?
The near-complete breakdown of international cooperation during the COVID-19 crisis prompted WHO member states to decide to revamp international law on disease outbreaks. The United States, Brazil, China, India, and other countries preferred amending the International Health Regulations (IHR), seen as a more technical document that legally binds all member states that do not opt out of the regulations. The European Union, a range of developing countries, and the WHO wanted a new pandemic treaty that would, in theory, carry greater political weight among governments that opt into it.
In 2021, WHO member states struck a compromise to negotiate amendments to the IHR and a pandemic treaty at the same time. That two-track process would bundle the amendments and treaty together as a package deal for the World Health Assembly to adopt in May 2024.
Diplomats struggled to negotiate simultaneously but separately the IHR amendments and a pandemic agreement, however. The parallel talks created headaches both for developing countries, which typically have smaller diplomatic missions, and for nations with larger delegations, given the complexity of coordinating across government departments. Neither track finished before the World Health Assembly convened in late May, and WHO member states continued negotiating the IHR amendments during the meeting.
Importantly, the World Health Assembly adopted targeted amendments to the IHR—a major feat in today's geopolitically divided world. As others have analyzed, the amendments preserved important features of the regulations and added provisions on financing and access to health products, such as vaccines. In contrast, WHO member states did not reach consensus and extended those talks for another year.
That failure could take the political wind out of the sails of the negotiations
Although adoption of the IHR amendments frees up diplomatic resources for negotiations on the Pandemic Agreement, the package deal of international legal reforms failed to materialize. That failure could take the political wind out of the sails of the negotiations because countries that prioritized the IHR amendments face less pressure to reach agreement on the treaty. Those changed political dynamics matter because WHO member states remain at odds on important issues concerning a pandemic agreement.
Many wealthier countries want the agreement to include legally binding obligations on a One Health approach that integrates human, animal, and environmental health efforts and on the rapid, open sharing of biological samples and genetic sequencing data (GSD) for pathogens of pandemic potential.
For developing countries, the top objective has been securing equitable access to health products such as vaccines, drugs, and diagnostics. One way to do so involves conditioning the sharing of biological samples and GSD on guaranteed access to such products—referred to as a pathogen access and benefit-sharing (PABS) system.
The IHR amendments did not include the One Health approach or a PABS system, and the amended IHR's provisions on health products are vague and limited relative to what has been agreed or proposed in the draft pandemic agreement.
Surprising Points of Convergence
Most multilateral treaties take years to negotiate. It is difficult for 194 governments to reach agreement on legally binding commitments concerning complex and consequential issues—commitments that will be difficult to change once made. Scholars have also argued that the rise of emerging powers makes gridlock in global negotiations more likely because parties are less unevenly matched than before. But countries have found ways around this gridlock in global health.
In the weeks leading up to the World Health Assembly, the INB extended the talks past deadlines and worked late into the night until the eve of the assembly. They agreed on about 80% (143) of the Pandemic Agreement's 177 paragraphs. Some agreed provisions are low-hanging fruit or either aspirational or vague. But the INB did forge agreements on contentious issues, such as intellectual property (IP) and financing.
IP has long been one of the most controversial issues that has divided higher and lower-income countries. In the Pandemic Agreement negotiations, WHO member states have supported the right to use flexibilities in international IP rules, and, going further, agreed to respect the use of such flexibilities. Developing countries wanted that additional commitment because governments of IP-exporting countries have exerted political pressure on developing nations not to use those flexibilities to increase access to IP-protected health products.
Financing has also been a sticky issue. Lower-income countries have perceived the push by higher-income nations for more investment in pandemic preparedness and response as a costly burden. Implementation of the IHR, for example, has suffered because the regulations did not address financing.
High-income countries could grease the wheels of compromise in the INB by making credible financing commitments, especially on One Health obligations that could be economically disruptive for wildlife or agricultural practices. But wealthier countries have been unwilling to accept legally binding duties to provide international financing, and the IHR amendments and pandemic agreement negotiations have repeated that pattern.
Even so, WHO member states have reached agreement on important financing issues. The amended IHR and consensus text for the Pandemic Agreement recognize the specific needs of developing countries, even if negotiators did not include the language of "common but differentiated responsibilities" such countries supported.
In addition, consensus text in the Pandemic Agreement commits governments to "maintain or increase domestic funding, as necessary" for pandemic preparedness and response, to "mobilize additional financial resources" to support other countries, and to "promote innovative financing measures" for pandemic strategies, all "subject to national and/or domestic law and available resources."
WHO member states also agreed to establish a coordinating financial mechanism that will operate under the amended IHR and the pandemic agreement. The mechanism seeks to improve the coherence, transparency, and efficiency of financial flows and to support countries' access to financing.
International pandemic funding comes from many sources, including national aid agencies, development banks, global health initiatives, multilateral institutions, and nongovernmental organizations. But such funding is disjointed, uncoordinated, and nearly impossible to track. Improving the efficiency of financing through the coordinating financial mechanism is critical given that the total envelope of development assistance for health is likely to decline in coming years.
WHO member states could still attempt to reopen the agreed text in the draft agreement, though political pressure will be high not to unravel carefully tied knots. Nevertheless, that the INB reached consensus on IP and financing issues is reason for cautious optimism that the resumed negotiations can cross other big chasms.
Where Deep Divisions Remain
Most of the thorny, unresolved issues concern access to and control of health products. Given that the Pandemic Agreement negotiations are the first major effort to achieve better access to health products in a binding treaty, it is no surprise that consensus has been elusive. But credible, reliable solutions for access have to be found if the Pandemic Agreement is to make good on the promise of more equitable pandemic governance.
WHO member states agree on the need to increase and diversify production capacities globally, but they disagree over how robust technology transfer obligations should be. Member states support investing in innovation, but do not agree on how assertive governments should be with pharmaceutical companies on IP rights when public money pays for research and development. Disagreements also exist over what should replace the Access to COVID-19 Tools Accelerator and what roles the WHO, global health initiatives, or regional organizations should play in procuring, financing, and distributing health products.
The Pandemic Agreement should treat pathogen access and benefit-sharing on an equal footing
Each of those issues are linked to the wrangling over a PABS system, which remains the lynchpin of any deal on the Pandemic Agreement. Negotiators have agreed that countries should share samples and GSD on pathogens of pandemic potential in a timely manner, and that countries sharing such samples and data should have access to technologies in return. Put differently, the Pandemic Agreement should treat pathogen access and benefit-sharing on an equal footing.
But divisions are stark on which benefits should flow, from whom and to whom, how to make the PABS system reliable and enforceable, and who should pay for it. For example, the proposal that 20% of real-time production of health products needed during pandemic emergencies should be sold or donated to the WHO for international distribution has provoked heated debate.
Before the World Health Assembly convened in May, the INB considered addressing those health-product issues after adoption of the Pandemic Agreement by negotiating a PABS-specific instrument. Parallel negotiations by states parties to the Convention on Biological Diversity on benefit-sharing linked to GSD have made it more complex for member states to finalize a PABS system for pandemic pathogens. Governments also considered whether they should handle the One Health issues through a separate instrument. But member states did not reach agreement on either of those PABS or One Health proposals.
Next Steps
The talks this month will focus primarily on the INB's process and methods of work, including changes to its leadership group. INB sessions over the coming year need to resolve a handful of technically complex and politically contentious issues, especially on health products and the PABS system.
In addition, the push to achieve consensus on the substantive issues before the World Health Assembly meeting meant that the INB spent too little time on the Pandemic Agreement's governance arrangements. WHO member states should consider including robust monitoring and accountability provisions to ensure that the treaty has meaningful impact and amounts to more than beautiful words on paper.
A strong, effective pandemic agreement is still needed for a safer, fairer world. Progress made to date suggests that creating such a world might be within reach.