This tracker is no longer being updated as of March 13, 2025.
For the second time in two years, the World Health Organization (WHO) has declared a public health emergency of international concern (PHEIC)—now in response to a surge of mpox cases in Democratic Republic of Congo (DRC) and neighboring countries.
The WHO's decision came on August 14—a day after the Africa Centers for Disease Control and Prevention (Africa CDC) announced its first public health emergency of continental security over the same crisis, which emphasized the inadequate global support the continent received during the global mpox emergency that ran from July 2022 to May 2023. In February 2025, the WHO voted to extend the PHEIC, in part due to the emergence of a new variant detected in the DRC.
The Africa CDC had called for 10 million doses by 2025 to respond to the multicountry outbreak, and DRC Minister of Health Samuel-Roger Kamba is reportedly seeking 3.5 million doses for his country alone. Bavarian Nordic stated in September 2024 that the company could produce as many as 13 million new doses of its mpox vaccine by the end of 2025 if it receives orders, and up to as many as 50 million doses over the next 18 months.
Based on official government statements and media reports, Think Global Health has identified the countries and companies that have pledged vaccine doses to Africa. This tracker was last updated on March 13, 2025.
Three Mpox Vaccines
As the global health community coordinates its response to the viral disease, it relies on three vaccines originally created to fight smallpox but repurposed during the previous mpox emergency, given that the viruses are related.
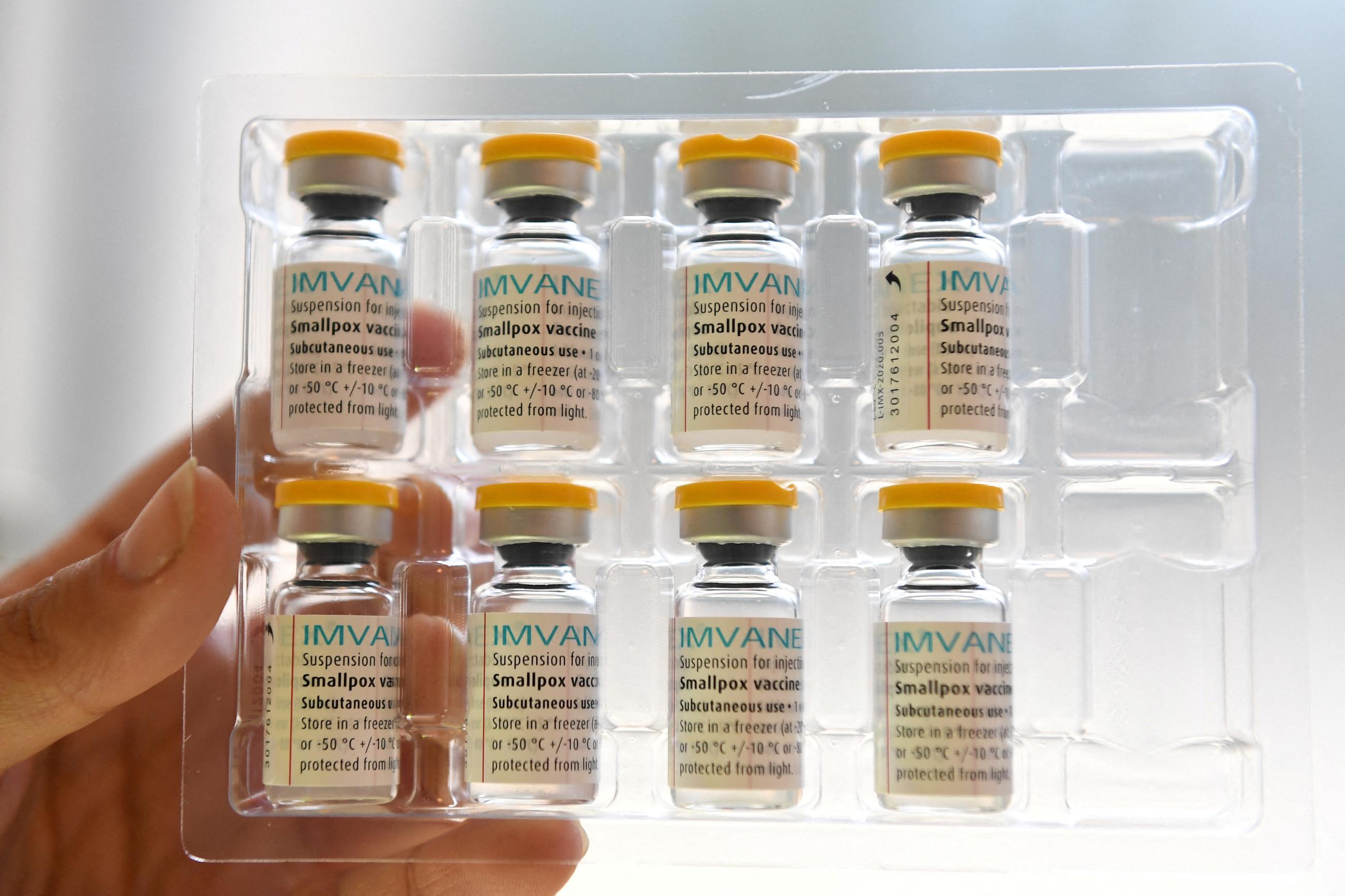
According to the U.S. CDC, the "best protection" against the mpox virus is two doses of the Jynneos vaccine, made by the Danish manufacturer Bavarian Nordic, which also goes by the names MVA-BN, Imvamune, and Imvanex. The LC16 single-dose vaccine produced by KM Biologics is yet to be internationally commercialized but has been approved domestically in Japan; in June, it received emergency authorization from DRC.
On August 29, 2024, the U.S. Food and Drug Administration expanded the approval of a third smallpox vaccine, ACAM2000 manufactured by Emergent BioSolutions, to prevent mpox disease. This vaccine has been shown to cause more side effects than the other two. The WHO recommends use of ACAM2000 only when other options are not available.
Until recently, LC16 had been the only vaccine licensed for children (in Japan), and minors account for 75% of mpox cases in DRC. On September 19, the European Medicines Agency (EMA) recommended extending the indication of the Jynneos vaccine to adolescents ages 12 to 17. In late October, Bavarian Nordic announced a clinical trial to expand the vaccine's approval for children ages 2 to 11.
According to the WHO, as of late August, none of these vaccine options had been tested in randomized controlled trials for mpox, the gold standard of gauging effectiveness—though some observational studies of Jynneos recorded real-world effectiveness during the previous emergency. The WHO has since announced its approval of Bavarian Nordic's Jynneos vaccine for prequalification status for adults and adolescents, and it recently granted the LC16 vaccine emergency use listing authorization, allowing major donors like Gavi and UNICEF to officially purchase the vaccines. The WHO had faced criticism after declaring the PHEIC for not granting this approval despite Bavarian Nordic's submission of safety and effectiveness data in 2023, and the organization is still reviewing ACAM200.
WHO is also recommending the use of a single dose—instead of two—to help stretch supplies in strained, but the agency emphasizes the need to collect further data on vaccine safety and effectiveness for this dosing strategy. Available data shows a small drop in effectiveness, WHO states.
Although the WHO has not issued official guidance on the use of booster shots (more than two vaccine doses) for Jynneos, the U.S. CDC stated it does not recommend them at this time. A study published in JAMA in October indicates waning antibody responses within 12 months of vaccination.
Gavi announced on September 18 that it would purchase 500,000 doses from the Danish pharmaceutical. Bavarian Nordic states the company could produce as many as 13 million new doses of its mpox vaccine by the end of 2025 if it receives orders, and up to as many as 50 million doses over the next 18 months "subject to regulatory approvals and market demand."
Vaccines Delivered
On August 27, the WHO reported the first delivery of mpox vaccine donations to Nigeria—10,000 Jynneos doses provided by the U.S. government that had been originally pledged in 2022. Nigeria donated 1,000 of these vaccines to Rwanda, which on September 17 became the first country to begin its vaccination campaign. On October 7, Rwanda also received 5,420 doses from HERA as the country now tackles broader health threats, including the outbreak of the highly fatal Marburg virus.
In September, the 200,000 doses pledged by the European Commission's Health Emergency Preparedness and Response Authority (HERA) arrived in DRC's capital Kinshasa, along with the 50,000 Jynneos doses pledged to DRC by the U.S. Agency for International Development (USAID) and the 15,460 doses donated directly by Bavarian Nordic.
On October 5, the DRC Ministry of Public Health and Prevention began administering its vaccines with the assistance of UNICEF to 11 health zones, starting in the province of North Kivu with frontline health workers, those in contact with mpox patients, sex workers, and immunocompromised individuals.
On November 6, the Africa CDC, working with CEPI, Gavi, UNICEF, and the WHO, announced that it had efficiently allocated an additional 899,000 doses of the vaccine to nine countries: Central African Republic, DRC, Ivory Coast, Kenya, Liberia, Nigeria, Rwanda, South Africa, and Uganda. 85% will go to DRC. After months of reported delays over liability concerns, the Africa CDC announced in December that the DRC would start to receive some of the 3 million pledged LC16 vaccines, the only one approved for children under 12, along with personnel from Japan to train health workers on the bifurcated needle administration involved for this vaccine. 50,000 doses from Japan are reported to now be in the DRC as of early 2025.
Congo's mpox response team initially reported that vaccine uptake and awareness was slower than expected, with approximately 50,000 people so far vaccinated against mpox in the Democratic Republic of Congo and Rwanda. By early November, both countries reported they had met or passed their vaccine targets.
Early in 2025, surges in violence around Goma in eastern DRC reportedly stalled mpox vaccination efforts. According to Africa CDC, the USAID funding freeze also reportedly disrupted DRC's mpox testing. USAID had been responsible for sample transport from outbreak hotspots to the central laboratory, according to the head of Africa CDC's incident management support team Ngashi Ngongo. In March, the Africa CDC reported disruptions had propelled a drop in weekly testing for mpox by 10%. At the same time, approximately 300,000 people have been vaccinated against mpox over the past 10 days in the western city of Kinshasa after health officials broadened outreach from contacts and target populations to anyone living in geographic hotspots.
In December, 11,200 doses, donated by the United States and facilitated by Gavi, were shipped to Nigeria. Following the US pledge in September to donate up to 1 million doses for Africa's mpox response, 305,000 of those doses—which include the 11,200—are to be coordinated through Gavi: 273,000 are currently doses planned for the DRC and 19,600 for Rwanda. Gavi has stated that it is still working with the US on the remaining 695,020 doses it has pledged. 10,000 vaccines previously pledged by Team Europe arrived in Uganda in January, which has since seen strong vaccine uptake. Health officials prioritized sex workers for the first vaccines, and according to Ngongo, 9,000 of 10,000 doses have been administered since February 1.
On February 11, the 200,000 vaccine doses, donated by Canada and facilitated by Gavi, arrived in the DRC. Officials will distribute the doses throughout the country according the Access and Allocation Mechanism (AAM) for mpox, a plan for allocation run by Gavi, the WHO and Africa CDC.
Pledges and Stockpiles
Pandemic agreement negotiations had put pressure on countries to donate given the widespread inequities in COVID-19 vaccine distribution. Many more vaccines than have been donated from high-income countries are likely currently available, estimated to be somewhere in the hundreds of millions. Bavarian Nordic declared that it had supplied more than 15 million doses globally during the mpox emergency that occurred from 2022 to 2023. In August, the pharmaceutical company announced a contract with an undisclosed European country to supply 440,000 doses of the Jynneos vaccine, and the United Kingdom (UK) announced it was purchasing an additional 150,000 doses on September 16.
The United States, which had delivered 60,000 vaccines to Africa as of October 1 and announced a donation of up to 1 million doses and $500 million at the United Nations General Assembly, deployed more than 1 million vials of the Jynneos vaccine nationally between 2022 and 2024. Although Biden administration officials would not confirm the current number of vaccines held in the U.S. stockpile in September, the Administration for Strategic Preparedness and Response (ASPR) stated that the government supply to be at 7 million vials in mid-2023. ASPR has also previously reported having 100 million doses of ACAM2000 in its stockpile.
Following Donald Trump's election in November, the Africa CDC asked the incoming administration for assurances that it would follow through on the previous administration's pledge to deliver the funding and mpox vaccines. Since the Biden Administration's pledge, only 61,200 doses have arrived in Africa, and the Trump Administration does not appear to have publicly commented. Ngashi Ngongo stated in a briefing in early February that $340 million of the pledge had been dispersed, and he said that Africa CDC is unaware of any vaccine delivery status changes amid USAID's dismantling.
Canada, which donated 200,000 doses, also has not disclosed its stockpile for national security reasons. The country entered a 10-year supply contract with Bavarian Nordic for its mpox vaccine valued at $470 million, with the majority set to be delivered in 2023. A low estimate of 2 million doses in its stockpile has been reported.
Japan, which confirmed its pledge of 3 million doses of its LC16 vaccine to DRC, has been estimated to have a supply of as many as 200 million doses of LC16, according to a WHO document from 2022. Although KM Biologics declined to comment to Reuters when pressed on this estimate, a Japanese health official said the 200 million was not accurate and would "confirm the size of the national stockpile."
Minors account for 75% of mpox cases in Democratic Republic of Congo
Clade I Spread
Even though mpox is endemic to DRC and parts of central and west Africa, cases have risen dramatically since January 2023. The virus breaks into two major ancestral groups of collections of strains, known as Clades I and II. Each clade has two subgroups (a and b).
Clade I is the cause of the new global emergency. In particular, Ia strains, which are endemic in DRC, have now spilled into the Central African Republic and Congo. Clade Ia is considered more severe and deadlier, especially among children, relative to what's been observed for Clade II, which sparked the previous emergency in 2022.
Clade Ib surfaced this year in DRC and appears to be spreading more easily between people, though this trait is unclear because of a historic lack of testing for the disease.
In February 2025, Africa CDC officials reported the detection of a new variant of clade 1a in DRC—raising concern as the older clade 1a virus is deadlier and more severe.
Since declaring the PHEIC, cases continue to spread to new countries on the African continent and beyond. Sierra Leone declared a state of emergency in January after reporting its second case, and cases of Clade Ib have been detected in China, France, Germany, Sweden, Thailand, the United Kingdom, the United States, and most recently in Brazil.
EDITOR'S NOTE: The tracker was updated to reflect the donation of MVA-BN "vials," one of which can correspond to a single dose intended for subcutaneous vaccination or five doses for intradermal vaccination. Spain's donation was corrected to reflect that the country had pledged 100,000 vials. This article was originally published on August 27, 2024.