More than 17,000 children in the United States and 300,000 children globally are diagnosed with cancer every year. In children, cancer develops when healthy cells multiply uncontrollably and tend to move to different body parts (metastasis). Leukemia, brain tumors, and solid tumors are the most common forms of pediatric cancers.
Like adults, children can develop cancer in any part of the body, but unlike adults, there are no lifestyle causes—such as smoking—that are known to cause cancer in kids. Childhood cancers are primarily caused by harmful changes in the normal developmental process and that may be linked to genetics. A medical history, including symptoms, and test-driven diagnostics followed by imaging technologies help diagnose the disease in youngsters.
State-of-the-art care and facilities are available in high-income countries (HICs), where the survival rate for pediatric cancer patients is more than 80 percent. But in low- and middle-income countries (LMICs), less than 30 percent of children with cancer survive. The most common reasons for low survival rates are delay in diagnosis, lack of access to reliable cancer treatment, abandonment of treatment, death from toxicity (side effects), avoidable relapse, and no or limited access to reliable, shared childhood cancer data.
In high-income countries, the survival rate for childhood cancers is more than 80 percent
Pediatric Cancer and Cancer Moonshot
Sharing cancer data to improve cancer prevention and treatment is one of U.S. President Biden's relaunched Cancer Moonshot goals—and if achieved, it will help advance pediatric cancer diagnosis and treatment in LMICS, not just in the United States. So far, under the original Cancer Moonshot project launched in 2016, the National Cancer Institute (NCI) has spent more than $1 billion supporting research on expanding personalized precision therapies, overcoming cancer's resistance to treatments, and finding new ways to treat pediatric cancer.
In order to come up with new cancer treatments for children, scientists often experiment using promising treatments for adults in children—with their parents’ consent. Sometimes, when chemotherapy and radiation—standard treatments for children with cancer—either stop working or don’t work at all, doctors look for other types of treatments that have been proven in adults. Immunotherapy, a cancer treatment that uses the body's immune system to attack cancer cells, is an example of an adult treatment that ended up working in children, too. For example, the FDA recently approved CAR-T cell therapy for Acute Lymphoblastic Leukemia (ALL) in children, teenagers, and young adults after a long time in clinical trials.
Several flagship Cancer Moonshot initiatives focus specifically on pediatric cancer. Still, adult cancer draws more attention and funding than pediatric cancer, especially in LMICs. To further the goal of data sharing in cancer research, the NCI has launched the Molecular Characterization Initiative for pediatric tumors. Under this program, the genetic material (DNA and RNA) in tumor and blood are analyzed to determine the most appropriate treatment plan for a particular patient and whether a child is eligible for a clinical trial. The samples from the cancer patient are sent to an accredited lab for analysis, and then the results are shared with their family, doctors, and researchers for future studies. Data analysis at the molecular level helps doctors identify the cause of individual cancers and choose the most effective and potentially least toxic treatment for a child. This voluntary state-of-the-art program is free for children, teens, and young adults across the United States who have newly diagnosed central nervous system tumors. There are plans to expand this program to soft tissue sarcomas and other rare tumors in the second half of 2022.
The NCI-backed Children's Oncology Group (COG), which constitutes more than 200 hospitals and institutions, currently offers the Molecular Characterization Initiative in the United States. The COG is the world's largest organization devoted exclusively to childhood and teenage cancer research. It unites more than ten thousand experts in childhood cancer across North America, Australia, New Zealand, and Europe to fight against it.
Recently published research suggested that the genomic landscape of childhood cancer is more complex than adult cancer. Genetic mutations are predominant drivers of pediatric cancers compared to somatic mutations that are more common in adults. In addition, alterations in the genetic material, like gene fusions and changes in the number of chromosomes (humans have 23 pairs of chromosomes), are more common in pediatric cancers. Understanding these pediatric cancer-related changes in the genome is critical for applying personalized therapies. To further understand the implications of these genetic modifications, the Blue Ribbon Panel (a team of leading cancer experts created by the National Cancer Advisory Board (NCAB)) has suggested ten transformative research recommendations for hastening Cancer Moonshot's goals. One such recommendation focuses specifically on pediatric cancer. To implement this recommendation, the NCI created the Fusion Oncoproteins in Childhood Cancers (FusOnC2) Consortium, a multidisciplinary network of investigators studying select fusion oncoproteins involved in childhood cancers, including Ewing sarcoma (the most common bone cancer) and acute leukemias with poor prognosis. The significantly advanced research coming out of this consortium could lead to new therapies for rare and aggressive pediatric cancers.
There is a big gap between the government-funded basic cancer research and the pharmaceutical industry-funded late-phase clinical trials
As the Cancer Moonshot mandate fosters greater collaboration, a related program, My Pediatric and Adult Rare Tumor Network (MyPART), works with advocacy groups to raise awareness about rare tumors among researchers. It also increases access to biospecimens—such as blood, cells, DNA, or protein—for rare tumor research and connects patients with investigators.
The Blue Ribbon Panel promotes expanding the benefits of immunotherapy to child cancer patients. Under this initiative, the Pediatric Immunotherapy Discovery and Development Network (PI-DDN) is created to identify and advance collaborative research opportunities for new, more effective immune-based therapeutic regimens (for example, cancer vaccines, cellular therapy, combinations of immunotherapy agents, etcetera) for high-risk pediatric cancers.
Beyond collaborating with multidisciplinary research teams, the Cancer Moonshot encourages partnerships between federal agencies and the pharmaceutical industry. One such public-private partnership, the Partnership for Accelerating Cancer Therapies (PACT), is an alliance between the National Institutes of Health (NIH), the Foundation for the National Institutes of Health (FNIH), and twelve leading pharmaceutical companies with the Food and Drug Administration (FDA) providing an advisory role. These research initiatives will maximize the benefits of immune therapies for all cancer patients. The PACT project also collects data from several hospitals that treat pediatric cancer patients.
Cancer prevention and early detection are other Cancer Moonshot program priorities. The term "prevention" refers to preventing children from experiencing later harmful effects of cancer therapies by exploring treatment options with less toxicity. For example, personalized precision medicine promises to reduce the side effects of chemotherapy and radiation treatments that can affect a childhood cancer survivor later in life. Early detection is also a goal.
Despite all of the resources available to pediatric cancer patients in the United States, there is a big gap between the government-funded basic cancer research and the pharmaceutical industry-funded late-phase clinical trials. This funding gap between the translational, preclinical, and early phase trials often wastes time. And time is a luxury that most pediatric patients don’t have. Organizations such as CueSearch are now prioritizing research funding for translational projects advancing swiftly from bench to pediatric clinical trials to the marketplace. Their goal is to support and prioritize innovative therapies with solid potential leading to quicker, effective, and less-toxic treatments, including novel, targeted therapeutics, immunotherapy, and combination therapies. CueSearch's careful funding has already resulted in the identification of over three hundred new, potential drug targets in nine types of pediatric cancers. A novel cell therapy to reduce toxicity caused by the standard Acute Myeloid Leukemia (AML) treatment while retaining the anti-leukemia benefits, and other promising treatments are in late clinical trials for brain tumors.
From 2015 to 2019, the average global 5-year childhood cancer survival rate ranged from less than 12 percent in Africa to 83 percent in North America
Impact of Cancer Moonshot Worldwide
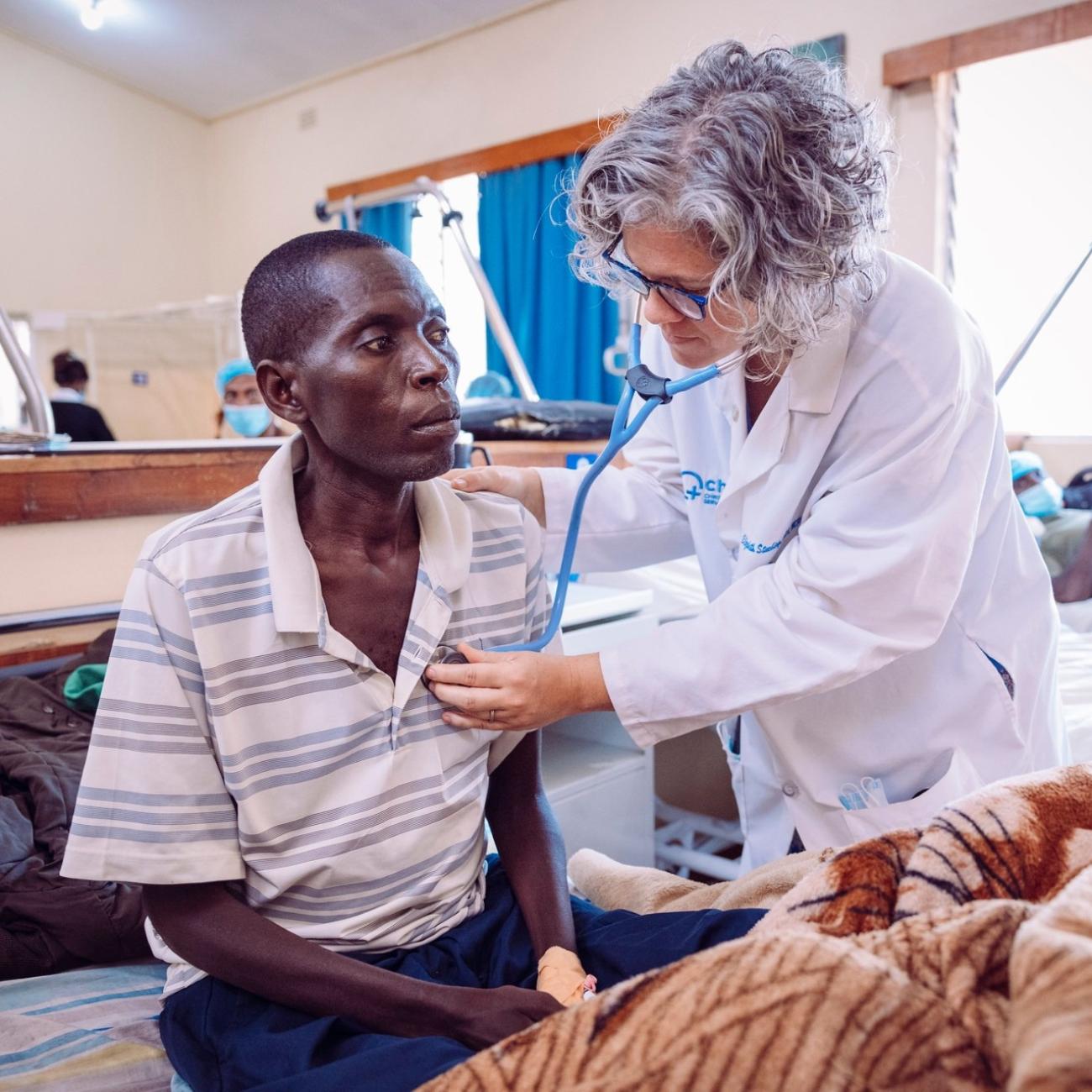
State-of-the-art treatments and knowledge gathered from networks such as the COG have resulted in better cancer management and improved survival rates in HICs over the past several years. However, in LMICs, slow progress in cancer-related research, initial diagnosis, and inadequate access to essential core technologies and treatments keep children with cancer from receiving life-saving treatment. For example, between 2015 and 2019, the average global five-year childhood cancer survival rate ranged from less than 12 percent in Africa to 83 percent in North America.
One challenge is that cancer faces severe stigma in many LMICs. The lack of awareness among policymakers, poor parental education, inadequate medical and research infrastructure, and unavailability or lack of access to cutting-edge medical technology adds fuel to this fire. Some LMIC governments are coping with other pressing health issues, such as maternal and child mortality due to infectious diseases, HIV, and malnutrition. But existing antiretroviral therapy programs for HIV could be adapted to accommodate pediatric oncology treatment programs, as well.
In addition, the lack of reliable data for pediatric cancer in LMICs is alarming. Social inequality also exacerbates the problem. Cancer patients whose parents have limited education are more likely to abandon treatment. This problem is persistent across LMICs in varying degrees—be it in Nigeria, Indonesia, or India. Cancer stigma is multiplied several times over for pediatric cancer predominantly due to cultural issues leading to inherent biases and the portrayal of childhood cancer in the mainstream media.
In LMICs, particularly in sub-Saharan Africa, pediatric oncology treatment abandonment is common due to cancer care's financial or travel burden and parents' low educational status. Infrastructure, capacity building, and adapting treatment to local capabilities promotes continual treatment. Facilities and equipment taken for granted in HICs, such as intensive care units, drug and chemotherapy agents, properly sanitized surgical instruments, and psychosocial support, are often in short supply in LMICs. Not only modern essential cancer medicines but even older, generic, and supposedly cheap cytotoxic agents are costly for many in LMICs. Fake medicines are an enormous concern in many countries. Belief in alternative therapies also significantly contributes to treatment abandonment.
Twinning—the pairing of a pediatric oncology unit in an HIC with a hospital in an LMIC—has been successful in many cases. For instance, one partnership between Georgetown University Hospital in Washington, DC, and Tikur Anbessa Hospital in Addis Ababa, Ethiopia, provides Ethiopian physicians with specialized pediatric oncology training—a critical step for a country with no trained pediatric oncologists—and it is building data management capacity and starting a telecare program to allow doctors in Addis Ababa to consult with Georgetown colleagues on complex cases.
In addition, with the robust penetration of technology in middle-income countries (MIC), free interactive online tools such as Cure4Kids and other AI-based tools are facilitating communication between health-care professionals across the globe. Another such effort by Switzerland's Société Internationale d'Oncologie Pédiatrique/International Society of Paediatric Oncology (SIOP), a multidisciplinary 2,600-member global society devoted to pediatric and adolescent cancer, collaborates with the World Health Organization (WHO) and other non-profit organiziations. Such support systems encourage collaboration between health-care professionals in LMICs and HICs.
The WHO Global Initiative for Childhood Cancer aims to increase the overall survival for six key childhood cancers (ALL, Burkitt's lymphoma, Hodgkin lymphoma, low-grade glioma, retinoblastoma, and Wilms tumor) to 60 percent globally by 2030 by integrating childhood cancer into national cancer control policies and capacity-building interventions including the development of national centers of excellence and regional satellites.
Miracles of modern science have saved millions of kids' lives and put smiles back on their parents' faces in HICs, but LMIC cancer kids have had only minimal support, so far. A better translation of oncology research into clinical care is the need of the hour. A marriage of modern computing, AI, and machine learning advances, and an open public dialogue can play a more prominent role in translational research. By enhanced sharing of data, one of the objectives of the Cancer Moonshot program, we hope to at least narrow the gap. As the world emerges from the worst of the COVID-19 pandemic, this is the time to bring the focus back to pediatric cancer. It is yet to be seen if Cancer Moonshot will be able to deliver on promises made or if it will remain a moonshot.